Nitrogen Use Efficiency (NUE) is defined as the number of pounds of N harvested with the crop divided by the number of pounds of N fertilizer applied.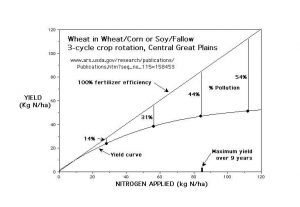
According to a widely cited article in the Agronomy Journal (1999, v. 91, p. 357-363) “Worldwide, NUE for cereal production is approximately 33%.” (42% in the developed world). “… affordability combined with the convenience of not having to apply N again during the growing season is attractive to farmers. In this regard excess N is applied as insurance, and because farmers are often overly optimistic concerning expected yields and yield goals. Because of this, the affordability of N in the developed world has led to its misuse and over application.” “Alternative N application strategies, specifically split applications (e. g., part pre-plant, part in-season) of N that are known to increase NUE, have not been widely adopted, largely because of the ease and affordability of applying more N than needed at or before planting. Agriculture’s focus in developed countries has been on maximizing yields per unit area …”
According to these authors, “There is no published research today where scientists have designed a package of practices specifically for high NUE.” “Excess N flowing down the Mississippi each year is estimated to be worth $750,000,000. At an average value of $490 per ton of N, $750,000,000 would comprise over 13% of the total value of N fertilizer ($5,480,356,000) applied in 1996 in the entire United States.” No wonder the huge dead zone exists in the Gulf of Mexico! Agronomists must change their mind-set and focus on NUE and the environmental consequences of both nitrogen and phosphorus fertilization practices much more seriously than has been done in the past. Agronomists are concentrated in land-grant colleges, originally established by the government to provide education aimed at crop productivity, and where funding from companies that profit from chemically intensive agricultural practices perpetuate the policy to “produce, profit, and damn the consequences.”
According to the 2004 and 2005 “Virginia On-Farm Corn Test Plots” the average yield for corn was 174 bushels per acre, grown with 162 pounds of chemical N fertilizer applied per acre. A bushel of corn weighs 56 pounds and contains about 15% moisture and about 1.4% N on a dry-weight basis. This means that about 116 pounds (174 * 56 * 0.85 * 0.014) of N was removed from each acre with the crop and the NUE was 72% (116/162.) What becomes of the other 46 pounds of nitrogen (162 – 116)? Most of the N not used by the crop ultimately ends up as nitrate in the groundwater, either directly or by oxidation of other N compounds like ammonia or NOx in the atmosphere, soil or groundwater. Roughly a third of the N might converted to nitrogen gas by denitrification, but unlike salt marshes and rice paddies, denitrification is not a major process in oxidized soils.
When animal waste (poultry litter, manure or municipal sewage sludge) is used as fertilizer, pollution is greatly increased because about half the N is not “crop available.” Approximately twice as much N is land-applied using animal waste as would be applied using conventional chemical fertilizer, to grow exactly the same crop.
I observed the land-application of sewage sludge in Northumberland County in March of 2004, and believe that the property owner and spreader adhered strictly to State policies, albeit in violation of Virginia Statute. Based on the submitted Nutrient Management Plan, 24,770 pounds of N were spread on 72.4 acres in accordance with Table 9-1 in the Virginia Department of Conservation and Recreation’s “2005 Nutrient Management Standards and Criteria” (“Standards”.) If chemical fertilizer had been used, 7,431 pounds of N would have been applied. Lime-stabilized sewage sludge is applied on the basis that 30% of the N is crop-available the first year, 10% the second and third years, and 5% the fourth year. The N application rate is determined by dividing the chemical fertilization rate by 0.3, or the amount of N available to the crop the first year (24,770 = 7431 / 0.3). 55% of the N is presumed to be crop-available over four years. So between 70% (if farmers do not reduce chemical fertilization in the next 4 years) and 45% (if farmers do reduce chemical fertilization in the next 4 years) of the applied N is released to the environment. There is no evidence that farmers routinely reduce chemical N fertilization in years following land application. This specific application caused at least 11,000 pounds of N pollution (2,200 50-pound bags of 10-10-10, or 30 bags per acre) to be released to the environment, compared to 2,080 pounds of N (7,431 * 0.28) that would have been released had chemical fertilizer been used.
In the case of phosphorus, the soil from 5 fields encompassing 55.9 acres tested “Very High” in phosphorus. This acreage should have received no phosphorus (and no sewage sludge) according to Virginia Statute 12VAC5-585-550.A “The applied nitrogen and phosphorous content of biosolids shall be limited to amounts established to support crop growth” and DCR policy as set forth in “Standards.” According to “Standards” the remaining 16.5 acres, based on soil tests, should have received no more than 1,151 pounds of phosphorus. In fact, 10,912 pounds of phosphorus were disposed (again, nearly 2,200 50-pound bags of 10-10-10.)
It is well known that “…much of the crop land in the Chesapeake Bay watershed is now considered ‘optimum’ or ‘excessive’ in phosphorus from an agricultural perspective and hence needs little additional phosphorus, from any source, to ensure that economically optimum crop yields are attained.” (A. N. Sharpley, Ed., Agriculture and Phosphorus Management: The Chesapeake Bay, 1999, CRC Press, p. 66.) According to the Mid-Atlantic Nutrient Management Handbook (p. 164) “… the critical level for soil test P … is around 30 ppm for Mid-Atlantic soils. If the test is below 30 ppm we would expect a profitable increase if we add P. However, if the soil test is above 30 ppm, no yield response is expected.” Almost all soils used for the disposal of animal waste test higher than 30 ppm P. Despite these facts, the State, EPA and CBF all continue to advocate the land-application of phosphorus-rich animal waste. Massive immediate nitrate pollution is the result, and the resulting high-phosphorus soils will “bleed” phosphorus into waterways for decades. Animal waste is better used as biofuel, simultaneously reducing greenhouse gas emissions and our reliance of foreign sources of fossil fuels.
Agronomists should concentrate on developing reliable, inexpensive timed-release fertilizers, so as to improve fertilization efficiency and assure that as much of the applied fertilizer as possible is consumed by the crop. Consider a corn crop fertilized with an efficient timed-release fertilizer. When they crop is mature and drying in the field at the end of summer, the fertilizer would have been consumed, and very little nitrogen and phosphorus would have been released to the environment, unlike conventional chemical fertilizers where over-application is necessary because a significant amount of the fertilizer washes out of the soil before the plants can use it. Animal waste, in contrast, and unavoidably, continues to be actively decomposed by microbes at the high soil temperatures, and almost all the nitrogen and phosphorus released constitutes pollution because there is no uptake by the mature crop. Efforts by agronomists to improve the uptake efficiency of nutrients in animal waste, or adjust animal diets, are futile and can never significantly reduce pollution from such inefficient sources of plant nutrition. Funding from municipal wastewater and poultry operators is the only reason agronomists “research” animal waste, when they should be concentrating their efforts in a productive direction, namely to improve the efficiency of chemical fertilization.