Nitrogen (N) chemistry is very complicated and many compounds are interesting and important. It is sufficient to know that most N on Earth occurs as an “inert” diatomic gas (N2) in the atmosphere. Organic and inorganic processes convert the N into forms usable by life. Because Earth’s atmosphere contains so much oxygen, again as a diatomic gas (O2), most N eventually ends up, sometimes via complex pathways, as nitrate (NO-3), the oxidized form of nitrogen found dissolved in surface water. Natural concentrations of dissolved nitrate are commonly less than about 1 milligram per liter (mg/l), or 1 part per million (ppm.) Higher values usually indicate an anthropogenic source, most often the excess fertilizer not used by plants. Values in excess of 1 ppm nitrate in drinking water are considered dangerous for infants. Although EPA’s Maximum Contamination Level is 10 ppm nitrate, values approximately half that have been implicated in cancers of the digestive system and other diseases.
Dr. Blake Ross (now retired) of Virginia Tech conducted a study of Household Water Quality in Northumberland County in 1997 and was kind enough to send me his raw data after he retired. The results from shallow wells (Nhist.pdf) confirm high concentrations of nitrate in shallow groundwater with an average nitrate concentration of 4.7 ppm. Subsequent studies of shallow water wells by SAIF water (www.saifwater.org) and by me confirm that the highest nitrate concentrations in groundwater in Northumberland County are always proximate to active agricultural fields.
A 2004 study of groundwater in the Delmarva Peninsula by the United States Geological Survey (USGS Circular 1228) documented an average nitrate concentration of 5.4 ppm and they stated (p. 2) “Concentrations of nitrate and herbicide concentrations in ground water of the Delmarva Peninsula are among the highest in the Nation.” and (p. 7) “Nitrate concentrations increase in shallow, near-surface ground water with increasing amounts of overlying agriculture.” The USGS (pubs.usgs.gov/circ/circ1316) recently concluded “… on average, ground water was found to contribute about 50 percent of the water and nitrogen to the streams and rivers that enter the Bay. The highest concentration of nitrogen in ground water occurred in areas overlain by agricultural land.” Any agency that lists runoff to the exclusion of groundwater as a source of pollution is suspect.
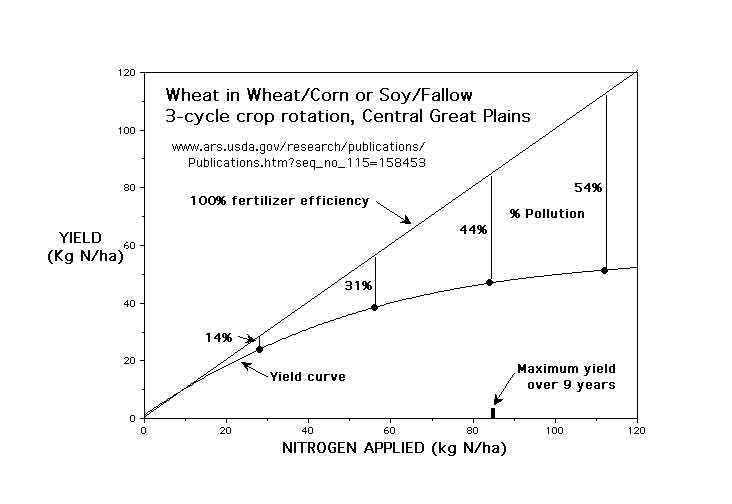
It is well documented that the Nitrogen Use Efficiency (NUE) by crops could be improved considerably. A typical “yield curve” demonstrates that as crop yield increases, the fraction of the nitrogen not removed from the field in the crop (mostly nitrate pollution) increases even faster.
Groundwater is commonly ignored as a source of pollution because it is “out of sight and out of mind.” Runoff is easily observed and is usually blamed for pollution. Rivers are much easier to analyze than is groundwater even though roughly one third of the water in a typical river is derived from groundwater (base flow.) In the Virginia coastal plain, approximately 2/3 of the 42 inches of annual rainfall is transpired by plants, or evaporates. Nearly all the remainder of the rainfall infiltrates the porous soils to the shallow water table and enters the groundwater. Runoff is rare except from impermeable surfaces like roofs and roads. The inland water table is always higher than sea level, so groundwater constantly flows “downhill” underground toward the nearest waterway as the distribution of aquifers permits. For each acre, given 14 inches of infiltration, 380,000 gallons of water is recharged each year (43560 square feet * 14/12 feet * 7.48 gallons/cubic foot). In order to maintain a steady-state groundwater level, the same amount of water discharges into waterways each year. At an average nitrate concentration of 5 mg/l, about 16 pounds of N (equivalent to three 50-pound bags of 10-10-10 fertilizer) is discharged from each acre each year unless some of it is consumed by riparian buffers or marshes. It is not uncommon for nitrate concentrations in domestic water wells near active fields to exceed 10 mg/l nitrate, the Maximum Contamination Level (MCL) permitted for drinking water by the EPA. The USGS found one sample from Delmarva containing 37 mg/l nitrate. The maximum I have ever found in Northumberland County is 15 mg/l, again, proximate to an active field.
Sadly, because groundwater flows relatively slowly, even if a field were allowed to return to forest, it will take decades for the high-nitrate groundwater to be flushed from the system. This is, of course, no excuse for inaction. Fertilizing as efficiently as possible should be mandated, but the only remedy for high nitrate groundwater that already exists is to intercept it at the coastline with a riparian buffer. The buffer should be at least 100 feet wide and eventually consist of mature trees, pruned of their lower limbs if a view is desired, with an overlapping leaf canopy, and bordered by marsh grasses if possible. The overlapping leaf canopy guarantees an overlapping root network. Unlike shrubs and grasses, mature trees have deep roots that can intercept the groundwater. The underground network of roots not only removes nitrate along with the transpired water, but promotes denitrification (called BNR or Biological Nitrogen Removal in a wastewater plant), or the microbial conversion of nitrate to harmless nitrogen gas. Marshes are extremely efficient in consuming nutrients, especially in converting nitrate to nitrogen gas, and should be encouraged wherever possible. The worst possible land use is an agricultural field tilled right up to the water. Nearly equally bad is a chemically maintained lawn bordered by a hardened shoreline. The Chesapeake Bay Act should be enforced (it is not being enforced) and strengthened to mandate a 100-foot buffer with no exceptions and no grandfathering. An efficient riparian buffer is cheap, and it is the only reasonable remedy for the high nitrate groundwater that already exists.